Critics complain that prescription drugs have contributed to a massive increase in health care spending in the United States. Some critics contend that drug prices are too high and propose lowering them through price controls. For example, the Prescription Drug Fairness for Seniors Act, currently before Congress, imposes a form of price controls on drugs sold to the elderly. Many complaints about price, and the laws those complaints spawn, are based on a misunderstanding of how the prescription drug market works. Following are some of the most common myths.
Myth No. 1: American Spend Too Much on Prescription Drugs. Per dollar spent, drugs offer a better return on health care spending than virtually any other health care option. Using prescription drugs often reduces or eliminates the need for costlier health care services. One recent study found that every dollar spent on drugs is associated with a $4 decline in spending on hospitals. The decline in total spending due to greater use of prescription drugs is particularly notable in the treatment of cancer, heart disease, Alzheimer's, AIDS and mental illness.
Myth No. 2: Drug Costs Are Rising Because of Price Increases. Drug cost increases are due primarily to nonprice factors, including increased volumes of prescriptions, record sales of new products and a changing mix of available products. Price increases to date have been relatively modest. According to a survey by IMS Health:
- Of a 14.2 percent increase in total drug costs in 1997, only 2.5 percent stemmed from price increases.
- Of a 15.7 percent increase in total drug costs in 1998, only 3.2 percent was caused by price increases.
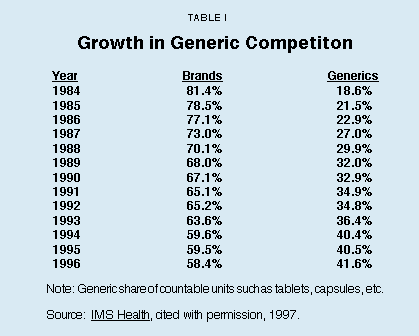
Myth No. 3: Americans Spend Too Much on Highly Advertised Brand-Name Drugs Instead of Purchasing Less-Expensive, Generic Drugs. Although generic drugs are supposed to be clinically identical to the brand-name drugs they replace, doctors and patients find that some effects are not uniform. Still, the market share of generic drugs more than doubled in a dozen years – from 18.6 percent in 1984 to 41.6 percent by 1996. According to some predictions, in the year 2000 about 70 percent of U.S. prescriptions will be filled by generic drugs.
Myth No. 4: Drug Prices Are Higher in the United States than in Other Developed Countries. Drug prices in the United States are not very different from prices in other developed countries.
- Using accurate pricing information, health economist Patricia Danzon has found that drug prices in Canada, Germany, Switzerland and Sweden are higher on average than prices in the United States.
- When "purchasing power parity," a means by which economists attempt to compare the price of goods in different countries, is considered, the Organization for Economic Cooperation and Development has found that Americans spend less per capita per year on drugs than do people in Germany or France and only slightly more than those in Canada and Italy – yet the other countries have controls.
Myth No. 5: Americans Could Reduce Their Drug Costs If They Paid the Same Prices as People in Less-Developed Countries. Critics of the U.S. system complain that consumers can buy drugs in Mexico for less than half their cost in the United States. Prices for the same drugs do differ in different countries, but Americans cannot get the newest drugs at Mexican prices for a simple reason. The research and development required to ready a drug for production can cost millions of dollars and take many years, but the cost of actually manufacturing a drug is usually small. Because manufacturers have discretion about pricing, the price may be close to production costs in poorer countries, which could not otherwise afford the drug, and higher in wealthier countries – more accurately reflecting the drug's value to patients. If all patients paid the lower price, there would be no money for research and development and no new drugs.
Myth No. 6: Seniors Are Overcharged for Drugs. Critics have produced a study that attempts to show that seniors pay twice as much for drugs as anyone else, but the study used inaccurate pricing information. Sixty-five percent of Medicare beneficiaries have some form of prescription drug benefit plan to help with their costs. In addition, seniors can buy drugs at a discount through AARP, private buying clubs, other organizations and discount pharmacies. A new study by Milliman & Robertson, the nation's leading actuarial consulting firm on health benefits, concludes that senior citizens could have comprehensive coverage for prescription drugs in addition to other Medicare benefits with virtually no increase in personal costs if private health plans were allowed to administer the benefits.
Myth No. 7: Price Controls Can Reduce Drug Spending. Attempts to drive down drug costs through price controls have two unintended results: (1) they encourage increased consumption of drugs and (2) they lead to the consumption of inferior drugs. Many European health systems with price controls spend more on drugs per capita than the United States spends, but Americans use newer and more appropriate medications. That is one reason Americans spend less time in hospitals when they are sick and have a higher quality of life than do Europeans.
Myth No. 8: Price Controls Would Reduce Drug Costs for Seniors. The Drug Fairness for Seniors Act would require drug manufacturers to offer all seniors the discounts now offered only to a very few government agencies, primarily the Veterans Administration. Yet, if the act became law, seniors would likely pay more, not less, for their prescriptions. At the earliest opportunity, drug manufacturers would steeply increase prices charged the VA. Further, to get the discount, pharmacies would have to do an estimated $15 worth of paperwork to prove to the manufacturer that each Medicare beneficiary was eligible – a cost pharmacies would pass on if possible. Thus seniors would end up with higher prescription drug bills.
Myth No. 9: We Can Have Price Controls without Rationing Drugs. If federal price controls for pharmaceuticals were adopted, an increase in consumption of pharmaceuticals would be inevitable and the government would then try to control the increase. That is what happened with erythropoietin (EPO), which is used to reduce anemia in kidney dialysis patients. Medicare, which pays for drugs for kidney dialysis, put a price control on EPO in 1994, rationed the amount patients could get and refused to cover patients with healthy blood cells above a certain level.
Myth No. 10: Price Controls Don't Affect Research and Development. Enactment of price controls or cost containment programs or both leads to decreased research and development spending simply because there is less money to be reinvested in R&D. Several countries that have implemented such programs have seen their pharmaceutical industries decline or go abroad. The United States, with no widespread price controls on drugs, produces by far the most innovative drugs.
Price controls represent not a mere extension of market pressure but a fundamental shift in values. Controls substitute a political process for the marketplace. For controls to work, individuals must be forced to adhere to governmental and bureaucratic decisions. This allows a few "experts" to decide what pharmaceuticals millions of physicians, pharmacists, medical researchers and patients "deserve" – and at what prices.
[page]In the past year, critics have complained that prescription drugs are contributing to escalating health care costs in the United States. Some also assail drug manufacturers, contending that drug prices are too high. They propose price controls as a way to lower drug prices. One bill currently before Congress, the Prescription Drug Fairness for Seniors Act, introduced by Rep. Thomas Allen (D-Maine), proposes a form of price controls on drugs sold to the elderly. President Clinton has proposed a prescription drug plan that would use private pharmacy benefit firms to negotiate drug prices with pharmaceutical firms and give Medicare the final say in what discounts the government can extract and what drugs seniors can use. Thus the Clinton plan hides government price controls behind a scrim of private sector contractors.
"Price controls have a consistent history: they don't work."
Price controls have a consistent history: they don't work. Whether they apply to air fares, gasoline, telecommunications or medicines, they discourage innovation, create shortages and fail to keep prices in check. Further, they harm the poor by making whatever is controlled more difficult and more expensive to obtain. Economist Ludwig von Mises once observed, "Economics does not say that isolated government interference with the prices of only one commodity or a few commodities is unfair, bad or infeasible. It says that such interference produces results contrary to its purpose, that it makes conditions worse, not better…."1
This paper examines a number of myths used to support the claim that drug prices are too high and that price controls are the best way to bring them down.
[page]"Dollar for dollar, drugs give us a better return on health care spending than virtually any other health care option."
Actually, we spend too little. Dollar for dollar, drugs give us a better return on health care spending than virtually any other health care option. Yet many private insurance plans have less coverage for drugs than for hospital or physician care, and many reimburse drug expenses at lower levels. Medicare, the federal government's health insurance program for the elderly, pays for very few prescription drugs. This practice encourages elderly patients and the doctors who advise them to seek physician and hospital services when less costly drug therapies would have been preferable.
In many cases, the use of prescription drugs has reduced the cost of other health care services. Even greater savings are possible. For example, one recent study found that every dollar spent on drugs is associated with a $4 decline in spending on hospitals.2 The decline in total spending due to greater use of prescription drugs is particularly notable in the treatment of cancer, heart disease, Alzheimer's, AIDS and mental illness. The following are some examples.
Cancer
- A Swedish study of the direct costs for treatment of cancer patients from 1985 to 1996 found that as the costs for outpatient care and drugs increased, inpatient care costs – and total direct costs – declined.3
- The use of granulocyte colony-stimulating factor (G-CSF), a biotech product that boosts white blood cell counts, leads to more rapid blood recovery and is associated with shorter, more predictable hospital stays after high-dose chemotherapy. Although G-CSF is expensive, it does not increase the overall cost of treatment.4
Heart Disease
- After high-risk coronary angioplasty, aggressive efforts to control blood clotting with a new biotechnology product reduces heart attacks and the reclogging of arteries, with little or no additional cost over the cost of therapy that otherwise would have been required.5
- A study released by the Agency for Health Care Policy and Research in September 1995 concluded that increased use of a blood-thinning drug would prevent 40,000 strokes a year, saving $600 million. In economic terms, the lifetime cost of a stroke exceeds $100,000, while the average annual cost of drug therapy and monitoring is $1,025.6
Alzheimer's Disease
- The cost of new drugs to treat Alzheimer's is offset by a reduction in the costs of care due to enhancement in cognitive functioning and the delay of more costly disease stages and settings.7
- One study found that pharmaceutical disease interventions that have minor effects on patients' cognitive status may result in large savings in the costs of caring for moderately to severely demented home-dwelling patients with Alzheimer's.8
AIDS
- A study of HIV-positive male and female patients in the Canadian province of British Columbia found that triple drug combination antiretroviral therapy with a protease inhibitor saved more in hospitalization costs than the cost of the drugs.9
- Combination drug therapy of three medicines – including a protease inhibitor – can reduce the AIDS virus in many patients to undetectable levels, enabling them to return to work and reducing the need for hospitalization. The annual cost ranges from $10,000 to $16,000, compared to the $100,000-a-year cost of treating advanced AIDS in a hospital.10
Mental Illness
- A new drug for schizophrenia reduced inpatient costs at Texas state psychiatric facilities by an average of $27,850 per patient per year compared to the cost of treating patients with more conventional drugs, according to a 1999 study.11
- Researchers at the National Bureau of Economic Research found that the total cost of treating acute depression fell by 25 percent during 1991-95, thanks to the introduction of new medicines.12
Prescription drugs also do things other therapies cannot, and the introduction of new drugs is closely associated with an increase in quality of life and economic productivity. Drugs not only save lives, they can also make lives happier and more productive.
[page]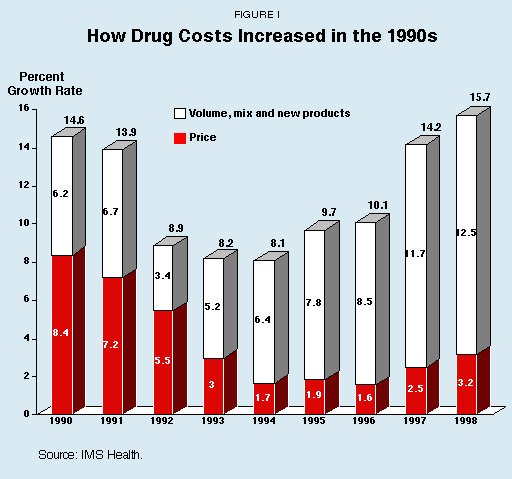
"Drugs costs are rising primarily because of nonprice factors."
Drug costs are rising primarily because of nonprice factors, including increased volume of prescriptions, record sales of new products and a changing mix of available products.13 Price increases have been relatively modest over the past 10 years. As Figure I shows, according to a survey by the leading prescription drug price and sales database information company, IMS Health:14
- Of a 14.2 percent increase in total drug costs in 1997, only 2.5 percent stemmed from price increases.
- Of a 15.7 percent increase in total drug costs in 1998, only 3.2 percent was caused by price increases.
According to the (less reliable) sampling methodology of the U.S. Bureau of Labor Statistics (BLS):
- Pharmaceutical prices rose 2.1 percent in 1996 and 3.6 percent in 1997, the lowest two-year average since the early 1970s.
- Manufacturers' drug price increases in 1995 were only 1.2 percentage points above the general inflation level of 2.5 percent and were 0.2 points below the index of medical inflation.
In addition, price indexes frequently do not adequately account for improvements in drug quality. For instance, new antidepressants carry an effectively lower price than older, cheaper antidepressants because they are safer, have fewer side effects, work better and can be taken once instead of three times a day. They also can offset more expensive health care services.
"Of a 15.7 percent increase in total drug costs in 1998, only 3.2 percent was caused by price increases."
Further, the reported price increases for drugs have been overstated, creating and sustaining the myth of rising drug prices and the contribution of price increases to total drug spending. A June 1995 study by the General Accounting Office (GAO) states that the government's Producer Price Index (PPI) for prescription drugs "has overstated drug price inflation substantially since at least 1984." According to the GAO study:15
- During the 1980s and 1990s the PPI showed that, on average, prescription drugs rose at triple the rate of inflation.
- However, flawed sampling exaggerated drug inflation by as much as 36 percent.
- In addition, the government's inflation index failed to reflect the savings generated by the increasing use of cheaper generic drugs.
Statistics used by supporters of price controls also ignore the price competition in the pharmaceutical industry that extends to both new and existing drugs. According to an April 1993 study by the Boston Consulting Group:16
- New drugs approved and launched in 1991 and 1992 were, on average, 14 percent cheaper than the bestsellers already on the market in their therapeutic classes.
- In some therapeutic areas, competition lowered prices even more. For example, four new ACE-inhibitors (types of cardiovascular drugs) launched in 1991 and 1992 had average introductory prices 36 percent below the leader in their therapeutic class.
Due in large part to "The Drug Price Competition and Patent Term Restoration Act of 1984" (Hatch-Waxman Act), which allowed generic drug companies to start manufacturing products before brand-name drug patents expired and to simply show that their copies of drugs were "equivalent" to a brand-name drug without conducting their own safety and effectiveness studies, the generic drug industry is a rapidly growing competitive force.
"Some predict that 70 percent of U.S. prescriptions will be filled by generic drugs in 2000."
Although generic drugs are supposed to be identical to the brand-name drugs they replace, doctors and patients find that the effects are not always uniform. While generics are generally acceptable, they are older technology and thus are not as clinically effective as newer medicines, particularly in the treatment of heart disease, depression and brain disorders. Still, as Table I shows, the market share of generic drugs more than doubled in little more than a decade – from 18.6 percent in 1984 to 41.6 percent by 1996. According to some predictions, in the year 2000 about 70 percent of U.S. prescriptions will be filled by generic drugs. The generic drug industry is expected to grow dramatically as the new century begins, as more than 200 drugs (with $22 billion in 1996 sales) lose patent protection.
[page]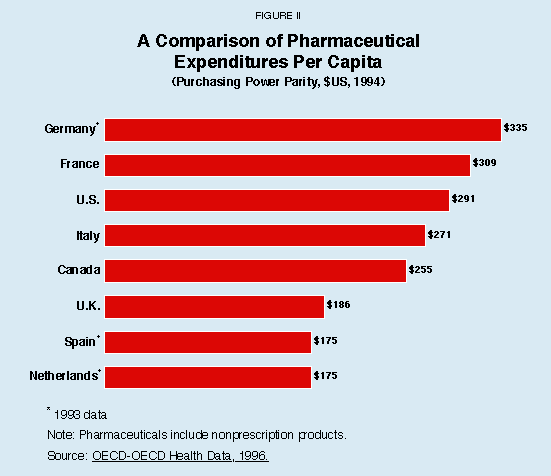
Drug cost comparisons between the United States and other countries that show U.S. prices are higher often are based on faulty or misleading price information. For reasons discussed in the next section of this paper, pharmaceutical companies, like makers of other products, charge different prices to different customers. In most comparisons, drug manufacturers' U.S. list prices are compared with list prices in other countries. Yet unlike in most other countries, transaction prices in the U.S. are generally lower than list prices, which are used as reference points for calculating discounts. This results in significant overestimates of U.S. prices.
"Most comparisons are based on list prices, but transaction prices U.S. generally are lower than list prices."
Price comparisons also are typically made by taking the average price of the top-selling brand-name drugs in America and comparing their average price in other countries. But the top-selling drugs in America are not the top sellers in other countries. Also, these comparisons exclude generic products that often have higher sales volumes and lower prices than brand-name drugs. A recent study of drug prices in nine countries by Patricia Danzon of the Wharton School, University of Pennsylvania, illustrates many of the difficulties of making international price comparisons.17 To make accurate comparisons, Prof. Danson determined the manufacturers' prices for between 187 and 484 products, depending on the countries being compared. She also converted the weights of the various products to U.S. measures and included generic drugs in the comparison. She found that drug prices in Canada, Germany, Switzerland and Sweden are higher, on average, than prices in the United States.
"Americans spend less on drugs per capita than do the Germans or French."
One of the difficulties in making international comparisons is that each country tends to consume higher quantities of the products that are relatively inexpensive in that country. In addition, international price comparisons are extremely sensitive to the price index used. A measure of drug costs that avoids these problems is the amount consumers spend on drugs adjusted for differences in the value of their currency – what economists sometimes refer to as "purchasing power parity." As Figure II shows:
- Americans spend less per year on drugs per capita than do Germans ($335) or French ($309) and only $20 more than Italians ($271).
- Annual per capita drug spending in Canada ($255) is only slightly less than in the United States ($291).
These countries and the others in Figure II, except the United States, all have price controls.
[page]Critics of drug companies complain that consumers can go to Mexico and buy drugs at less than half their cost in the United States. Such critics often contend that price controls could bring U.S. drug prices down to Mexican levels.
Prices for the same drugs do differ in different countries, but Americans cannot have access to the newest drugs by paying Mexican prices for a simple reason. The research and development required to bring drugs into production can cost millions of dollars over many years, but the cost of manufacturing most drugs is small. Because manufacturers have discretion in pricing, their prices may be closer to production costs in poorer countries, which could not otherwise afford the drug, and higher in wealthier countries – more accurately reflecting the drug's value to patients. If patients in every country paid the lower price, there would be no money for research and development and no new drugs.
"If all patients paid the lower prices charged in poor countries, there would be no money for research and development and no new drugs."
Most studies that compare international drug prices fail to compare income differences across countries. Those that do show that, in most countries, patients use higher quantities of drugs that are inexpensive for that nation. That is, people take the drugs they can afford. Prices for the same drugs do differ in different countries because in poorer countries, drug firms simply try to cover their costs or earn modest sums while they earn larger profits in wealthier nations.
Only one drug in 1,000 ever makes it to market. Much essential research and development never produces a usable drug. Prices also reflect the need to attract investment and earn profits for future research and development activities. Of all drugs that are introduced, three in 10 must be hugely successful to cover the costs of more marginal drugs and failures along the way.
As with computers, cars, telecommunications and other cutting-edge products, new pharmaceuticals are introduced in developed countries at higher prices, so consumers in those countries gain the first benefits but also pay a higher portion of the development costs. Nonetheless, new pharmaceuticals tend to be introduced around the world more rapidly than other technologies. For example, the costly antiviral AIDS drug AZT found its way into Africa within four to five years of its introduction in the United States. When scientists discovered that AZT often could prevent the transmission of AIDS from mothers to babies, this new use was adopted widely in Africa for pennies a dose at about the same time it was introduced in the United States.
[page]Supporters of Rep. Allen's Prescription Drug Fairness for Seniors Act have produced a study to show that drug companies charge seniors more than they charge anyone else, but the findings are based on inaccurate pricing information.18 The study claims that seniors in Washington, D.C., pay nearly 100 percent more for drugs than do managed care companies. The claim is based on the assumption that the prices HMOs pay for drugs are equivalent to prices in what is known as the Federal Supply Schedule (FSS). However, very few government agencies, primarily the Veterans Administration, get the FSS deep discount on prescription drugs. HMOs do not get FSS prices. Neither does the government, except for this special case. According to the General Accounting Office (GAO), "…many FSS prices are more than 50 percent below nonfederal average manufacturer prices. But companies have been willing to give federal purchasers such low prices because they consider the FSS to be a special, limited category of pricing that affects no more than about 2 to 3 percent of total dollars in domestic pharmaceutical sales."19
Another GAO report concluded that the typical "best price" paid by HMOs and hospitals was discounted 14 to 15 percent.20 A report by the Congressional Budget Office (CBO) put the discount at 19 percent.21 Sixty-five percent of Medicare beneficiaries have some form of prescription drug benefit plan, and seniors can buy drugs at a discount through AARP, private buying clubs, other organizations and discount pharmacies.
"Senior citizens could have comprehensive drug coverage with virtually no increase in personal costs if private health plans were allowed to administer the benefits."
The federal government itself creates problems for seniors. A new study by Milliman & Robertson, the nation's leading actuarial consulting firm on health benefits, concludes that senior citizens could have comprehensive coverage for prescription drugs in addition to other Medicare benefits with virtually no increase in personal costs if private health plans were allowed to administer the benefits.22 The study finds that private health plans have the ability to eliminate much of the waste and inefficiency in Medicare and apply the savings to the cost of prescription drugs not currently covered.
[page]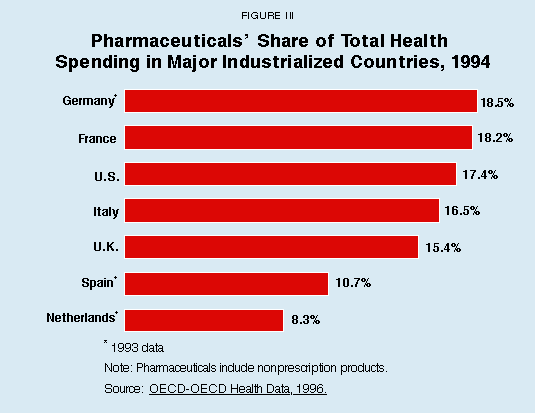
Proponents of price controls argue that cuts in drug prices will lead directly to cuts in total drug spending. In fact, the opposite will occur. A 1993 study by Heinz Redwood and a 1994 study by David Gross comparing international pharmaceutical spending controls found that:
- While price controls produced lower prices, they did not reduce total pharmaceutical expenditures (price times volume) nor did they contain total health care spending.23
- Moreover, pharmaceutical expenditures grew faster in countries with price controls than in the United States.
"Price controls encourage increased consumption of drugs and the consumption of inferior drugs."
Price controls usually are part of a government-run program that provides consumers with prescription drug benefits. Attempts to drive down the drug costs through price controls have two unintended results: (1) they encourage increased consumption of drugs and (2) they lead to the consumption of inferior drugs.
Many European health systems spend more on drugs per capita than is spent in the United States, but Americans use newer and more appropriate medications for such diseases as depression, high cholesterol, high blood pressure and cancer. That is one reason Americans spend less time in hospitals when they are sick and have a higher quality of life than Europeans.
European and Japanese consumers face an entirely different basket of pharmaceutical products than do Americans.
- The U.K. National Heart and Lung Institute noted that nearly 90 percent of Europeans who have experienced heart failure and who should be receiving ACE-inhibitors are not, despite the availability and demonstrated ability of these drugs to prevent second heart attacks and left ventricle dysfunction.
- Ninety percent of heart failure patients in France were not receiving an ACE-inhibitor, at a potential cost of 16,000 lives and $528 million over four years.24
Europeans are more likely to consume drugs that could never be approved by the U.S. Food and Drug Administration, which despite its reputation for regulatory delay is still the gold standard for drug approval worldwide.
However, it is Japan that demonstrates most vividly how reducing drug prices through controls affects health care quality. The Japanese government has in recent years cut prices of drug products from global pharmaceutical firms by 50 percent. It allows local drug companies to go to market with new drugs at higher prices but cuts the prices in later years. Patients do not pay for drugs directly; doctors make most of their money by filling their own prescriptions. Japanese doctors often load their patients with prescriptions, marking up the prices allowed by law and pocketing the difference. On average, a doctor prescribes 13 different medications to people under his care.25 As a study by Tom Thomas, an economics professor at Emory University, points out, the vast majority of Japanese medications marketed and prescribed are useless.26 Unable to obtain the best drugs to treat their diseases, Japanese consumers are sicker and spend more on other types of health care.
Price controls distort the quality of care in many ways, not least by shifting both the production and availability of medications towards older and less innovative compounds. The result is that people cannot have access to the most cost-effective medications and are forced into hospitals.27
"Price controls directly drive up total spending on drugs."
So consumers in countries with price controls often wind up consuming many more drugs that are less effective or totally ineffective than more innovative products. These countries tend to spend more on drugs as a percentage of their health care dollar, as Figure III shows, but they get less value in the process. Either way, price controls directly drive up total spending on drugs.
[page]The Allen bill would force pharmaceutical firms to give pharmacies the same discount for drugs bought by those on Medicare that the Veterans Administration gets under the Federal Supply Schedule. Supporters say the proposal is market-based and would make drugs more affordable; in fact, it would impose price controls and would almost certainly raise the cost of drugs for most seniors. Further, it would not extend prescription drug coverage to those who lack it.
"If the Drug Fairness Act became law, it would drive up prices for seniors."
Why would drug prices for seniors rise if the Drug Fairness Act became law? First, at the earliest opportunity drug manufacturers would increase steeply the prices in the Federal Supply Schedule. Further, pharmacies would have to prove that each Medicare beneficiary was eligible for the discount, since drug manufacturers would want to limit their financial exposure. According to Mick Kolassa, a professor in the College of Pharmacy at the University of Mississippi and an expert on drug prices, the paperwork would cost about $15 per prescription to process and collect.28 The pharmacy would pass this cost on, if at all possible. Thus seniors would likely end up paying more for prescription drugs than before.
In addition, price controls mandated by the Allen bill for one group of seniors would drive up costs for many other consumers. This has been the experience in the past. For example, in 1990 Congress decided to require drug manufacturers to give state Medicaid programs rebates for outpatient drugs based on the lowest prices they charged other purchasers. Because of the size of the Medicaid market, many drug manufacturers sought to cope with the requirement by simply reducing or eliminating the discounts to other purchasers. The result: drug spending went up in the private sector.
According to the GAO, the larger the government market, the greater the economic incentive for manufacturers to raise schedule prices to limit their losses. Since about 65 percent of seniors now have some form of private drug coverage, imposing price controls now would have an effect similar to what happened with Medicaid in 1990.
[page]If federal price control-based legislation for pharmaceuticals were adopted, the government effort to control the resulting rise in consumption would lead the government to decide who got what drugs.
Consider the Medicare program's handling of erythropoietin (EPO), a biotechnology product used to control anemia in kidney dialysis patients. (Medicare pays for drugs that are part of overall therapy such as cancer care, bypass surgery and diabetes management. Prescription drugs are not covered except when available through HMO plans.) Dialysis patients are healthier and longer-lived with EPO than without it. In 1994, to limit its expenditures for EPO, Medicare put a price control on the drug, rationed the amount patients could get and refused to cover patients with healthy blood cells above a certain level.
Subsequently the number of people who died in the program increased and people with healthy blood levels wound up getting sicker and spending more time in dialysis and in hospitals. It took five years of lobbying and administrative review to get Medicare to loosen its chokehold over the lives of diabetics.29
Drug rationing in state Medicaid programs also has been strongly associated with a huge spike in hospitalizations and doctor visits. Whatever money was saved on drugs was more than offset by the cost of the untreated illness.30 Price controls would institutionalize this perverse consequence.
"Drug rationing in state Medicaid programs has been associated with a huge spike in hospitalizations and doctor visits."
Further, a prescription drug benefit would invite bureaucrats to decide what drug the elderly could take and to switch drugs without patients' consent. Government-mandated drug switching interferes with the doctor-patient relationship and may compromise the health of seniors. Medicaid's price controls have been combined with such approaches to controlling the inevitable rise in costs associated with regulation.
- A 1991 study published in the New England Journal of Medicine found that when New Hampshire restricted the number of prescriptions reimbursed by Medicaid, the elderly entered nursing homes at a rate more than 60 percent greater than in a control state.
- Although drug utilization fell 35 percent, nursing home admissions rose 60 percent and overall health care expenditures increased.
- When the restrictions were lifted, nursing home admissions decreased.31
- A 1994 study in the New England Journal of Medicine by the same authors found that New Hampshire's prescription drug caps saved an average $57 per year on drugs for schizophrenia patients – but added $1,530 per year in costs for visits to mental health clinics and emergency rooms.32
Drug switching and rationing can harm the elderly because seniors can react differently to medications than do younger people. This is particularly true for medications used in treating depression, Parkinson's disease and high blood pressure. A change made to save money may force a senior into a nursing home or hospital. Yet advocates of a new drug entitlement program are the most aggressive supporters of forced substitution of generic medications by the government.
[page]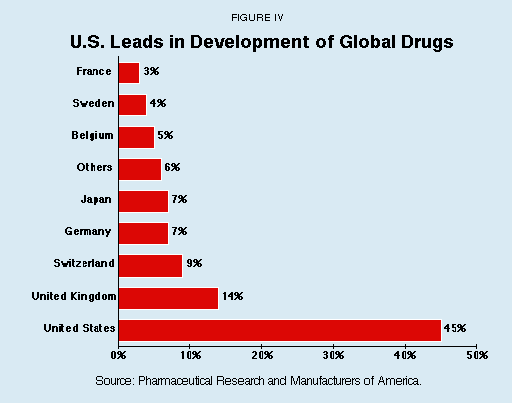
"The U.S. with no widespread price controls, develops a high percentage of the world's innovative drugs."
Rep. Henry Waxman (D-Calif.) is a prominent critic who claims that government efforts to control drug prices have no effect on research and development. However, experience with price controls belies his assertion. Enactment of price controls or cost containment programs or both decreases research and development spending because there is less money to be reinvested in R&D. Several countries that have implemented such programs have seen their pharmaceutical industries decline or go abroad. As Figure IV indicates, the United States, with no widespread price controls on drugs, develops a high percentage of the world's most innovative drugs.
- The United States developed 45 percent of all drugs marketed worldwide in the 1990s.
- The United Kingdom developed 14 percent of all global drugs and no other country developed more than 9 percent.
Why Price Controls Hurt Innovation. Whereas U.S. companies often have their eye on the worldwide market when they start to develop a new drug, European and Japanese companies produce largely local products. Their minor innovations are "either directly imitative of existing products or [are] unsafe products that cannot clear regulatory hurdles in many nations, or are products that fill minor local niches."33 Indeed, "of the top 50 products (by value) in each of four European countries in 1993, 10 of those in Italy and France were classed as useless, compared with six in Germany and none in the U.K."34 In France, lax standards to ensure a new drug's effectiveness also have contributed to the development of drugs that were safe but of dubious therapeutic value; such drugs accounted for up to 20 percent of total sales.35
What has happened in one of the largest prescription markets – cardiovascular products – illustrates that countries with price regulation tend to be less innovative.36
- In the 1980s nine different beta blockers were developed in the United States and the United Kingdom, 14 in France and 30 in Japan.
- Today low prices in Japan and France have discouraged firms in those nations from discovering major new products, and the United States has become the world's leader in developing new cardiovascular therapies, having surpassed the U.K. in the early 1980s.
Another way to assess the influence of price controls on innovation and global competitiveness is to examine recent measures in countries that have had price controls over the years. As the following overview suggests, different types of price and product regulations appear to reduce investment in innovative medicines.
"Ten of the top 50 drug products in Italy and France were classed as useless."
Case Study: United Kingdom. The Pharmaceutical Price Regulation Scheme (PPRS), in force since 1977, regulates profits and return on capital and explicitly encourages innovative research with higher profit margins and higher introductory prices. As a result, United Kingdom drug prices have been higher than the European average. Rates of return are negotiated and regulation extends only to home market sales. In fact, higher rates of return are provided for "export-oriented" firms. As a result, Britain's pharmaceutical industry was highly innovative and profitable during the 1970s and 1980s.
However, as cost containment became paramount, the policy of promoting innovation through higher prices and profits was dramatically altered. First, the National Health Service (NHS) tried to keep costs in check by limiting how much doctors could prescribe. More directly, the British government reduced profit rates by restricting price increases to less than the rate of inflation. As a result, return on capital in the U.K. pharmaceutical industry has declined to 10 percent, less than half of the 21 percent that is allowed under the PPRS. Domestic profits have declined to 4.5 percent of sales.
In the past, the British government imposed a 2.5 percent rollback of all drug prices, banned access to certain new drugs and imposed a cut in the prices and profits of innovative drugs. Companies exceeding negotiated rates of return must pay fines and reduce prices. According to a research analyst with the Association of the British Pharmaceutical Industry, British drug concerns reacted by shifting nearly $1 billion a year in R&D to their U.S. operations.37
The impact of these changes has affected innovation overall. In 1988 three of the 10 best-selling new products worldwide came from the United Kingdom. By 1992 the U.K. had only one of the top 10. Today, U.K. companies have no drug among the top 10. The United States has all 10 best-selling drugs worldwide.
Further, the PPRS has a built-in bias towards larger firms. Biotechnology companies find it difficult to attract investors because the financial rewards the PPRS allows for large-company R&D (a maximum of 21 percent return on capital) are not high enough for most small companies, which depend on breakthroughs to offset the risks of drug development.
"The United Kingdom had three of the 10 best-selling new drugs worldwide in 1988 — but it has none today."
Case Study: Germany. The German Health Ministry initiated efforts to control drug costs in 1989 by limiting what public health plans paid for drugs and which drugs would be eligible for reimbursement. If a drug's price exceeded the government limit, the patient had to pay the difference. In 1993 the government mandated a 5 percent cut in drug prices and slashed what the German health authority (the Krankenkassen) could spend on drugs by nearly 10 percent. Doctors have been placed on drug budgets and if they go over have to pay the excess out of their own fees. Sales fell at the seven largest research-intensive drug producers by 16.5 percent, while generic sales increased by 36 percent.38 As a result, at least 126 pharmaceutical firms cut their R&D investment, 40 percent by 10 to 30 percent and 22 percent by a third or more.
Case Study: France. The Ministry of Health in France controls the introductory price of each product, controls subsequent price increases and holds down prices regardless of value. As a result, France has some of the lowest drug prices in Europe. It also has one of the worst records in developing innovative products that can compete globally.
The most successful companies in France are firms that produce and market copycat drugs or mere palliatives. Innovators are punished with artificially low prices that favor companies that produce low-price drugs without regard to their drugs' therapeutic value.
The Rationale for Price Controls. The conventional wisdom is that price controls encourage companies to focus their resources on developing a blockbuster.
As this overview suggests, such a convention is not wisdom at all. Price regulation rewards incrementalism and punishes risk taking, particularly if prices and access to new drugs are regulated. Without innovation, a country's pharmaceutical industry cannot compete globally and its biotechnology industrial base cannot develop rapidly. That is one reason the European Community is considering ways to do away with price controls.
[page]The Clinton administration's proposal to control prescription drugs for the elderly has much in common with the French system of setting pharmaceutical prices and reducing the price of innovative drugs, as well as the German system of deemphasizing innovation in favor of generic drugs. Under the administration proposal, private U.S companies that administer drug benefits for the elderly and individual seniors could buy drugs at prices negotiated for the entire program by the federal government.
Is it possible that such price regulation in the United States won't discourage innovation? After all, the industry has gone through three years in which price increases have declined and even stagnated after discounts, price freezes and generics are taken into account. Managed care companies increasingly favor lower-priced drugs and now examine the cost-effectiveness of new drugs before paying for them. In addition, the industry has paid nearly $1 billion a year to the federal government in Medicaid rebates. Yet R&D spending has increased, not decreased. If both the pharmaceutical and biotechnology industries still are thriving and investing in innovative new products, why would government regulation over the prices of those new products make any difference?
"Because of the time lag between drug discovery and introduction, the market will not feel the full impact of the government price control policy for years."
First, because of the time lag between drug discovery and introduction, the market – physicians and patients in particular – will not feel the full impact of the policy for years. The initial cuts would come in the riskiest research for the hardest-to-treat illnesses. Then the market value of pharmaceutical and biotechnology stocks would decline. As cash flow declined, so too would R&D. For biotechnology companies, the decline in cash flow – and risk capital used for R&D – is dollar for dollar. Only breakthrough products yield robust earnings. A study by Henry Grabowski and John Vernon estimates what would happen if the government cut the price of breakthrough drugs by 23 percent – essentially establishing a break-even rate of return on R&D investment: cash flows for the best-selling drugs and biotechnology therapies would fall below the total cost of R&D. The result, particularly for biotechnology companies, would be devastating.39
Second, under the president's plan, the government would cover up to 50 percent of all prescription drugs purchased. As the largest purchaser of drugs, it would have immense, near monopsonistic power to force prices down, extract discounts and pay only for drugs that met its terms. Government could single-handedly create far more pricing pressure than any number of managed care organizations.
Finally, price controls represent not merely an extension of market pressure but a fundamental shift in values. Controls substitute a political process for the marketplace. For controls to work, individuals would have to adhere to governmental or bureaucratic decisions. Decisions by physicians, pharmacists, medical researchers, companies and patients would be replaced by those of a few "experts."
"Price controls represent not merely an extension of marketplace pressure but a fundamental shift in values."
Price controls supplant market forces and replace good health with cost containment as a goal. Lower prices send a signal that innovation will not be rewarded with higher returns. By limiting rewards and discouraging investment, price controls limit innovation. As Dr. Judith Wagner, a senior associate with the Health Program at the Office of Technology Assessment has noted, "an administrative process for controlling drug prices would add a new source of uncertainty – one that would not be resolvable until all the money has been spent. Consequently, investors would be more hesitant to commit early R&D money…."40 More recently, in discussing the Clinton drug plan, Barbara Ryan, one of Wall Street's leading pharmaceutical industry analysts, noted that "the specter of significant price controls has been sufficient to substantially reduce the appeal of drug stocks to investors. We expect this to persist throughout the election process in 2000. Drug price controls would substantially reduce pharmaceutical company investment in R&D, which would effectively stall the progress made in significant therapeutic advances. There is a direct relationship between profitability and the willingness to invest in R&D."41
NOTE: Nothing written here should be construed as necessarily reflecting the views of the National Center for Policy Analysis or as an attempt to aid or hinder the passage of any bill before Congress.
[page]- Ludwig von Mises, A Treatise on Human Action (New York: Ludwig von Mises Institute, 1997).
- F. Lichtenberg, "The Effect of Pharmaceutical Utilization and Innovation on Hospitalization and Mortality," National Bureau of Economic Research, Paper No. 5418, January 1996.
- Tennvall G. Ragnarson and G. Karlsson, "Cancer Treatment in Sweden – Costs of Drugs, Inpatient and Outpatient Care from 1985 to 1996 and Cost-Effectiveness of New Drugs," Oncology, Vol. 37, No. 5, 1998, pp. 447-53.
- S. M. Lee et al., "Recombinant Human Granulocyte Colony-Stimulating factor (Filgrastim) Following High-Dose Chemotherapy and Peripheral Blood Progenitor Cell Rescue in High-grade Non-Hodgkin's Lymphoma: Clinical Benefits at No Extra Cost," British Journal of Cancer, Vol. 77, No. 8, April 1998, pp. 1294-99.
- D. B. Mark et al., "Economic Assessment of Platelet Glycoprotein IIb/IIIa Inhibition for Prevention of Ischemic Complications of High-Risk Coronary Angioplasty," Circulation, Vol. 94, No. 4, August 15, 1996, pp. 629-35.
- Secondary and Tertiary Prevention of Stroke Patient Outcome Research Team: 9th Progress Report, paper delivered at Agency for Health Care Research, September 1995.
- P. J. Neumann et al., "Cost-Effectiveness of Donepezil in the Treatment of Mild or Moderate Alzheimer's Disease," Neurology, Vol. 52, No. 6, April 12, 1999, pp. 1138-45.
- R. L. Ernst et al., "Cognitive Function and the Costs of Alzheimer's Disease. An Exploratory Study," Journal of the Archives of Neurology, Vol. 54, No. 6, June 1997, pp. 687-93.
- A. H. Anis et al., "Modelling the Potential Economic Impact of Viral Load-Driven Triple Drug Combination Antiretroviral Therapy," Pharmacoeconomics, Vol. 13, No. 6, June 1998, pp. 697-705.
- Ibid.
- W. H. Reid, "New vs. Old Antipsychotics: The Texas Experience," Journal of Clinical Psychiatry, Vol. 60, Supp. 1, 1999, pp. 23-25 and pp. 28-30.
- R.G. Frank et al., "Price Indexes for the Treatment of Depression," in Jack Triplett, ed., Measuring the Prices of Medical Treatments (Washington, D.C.: Brookings Institution Press, 1999).
- IMS Retail and Provider Perspective, IMS Health, Scottsdale, Ariz., 1998.
- Ibid.
- General Accounting Office, "Prescription Drug Prices: Official Index Overstates Producer Price Inflation," Letter Report, April 28, 1995, GAO/HEHS-95-90.
- The Value of Pharmaceuticals (New York: Boston Consulting Group, 1993).
- Patricia Danzon, "The Uses and Abuses of International Price Comparisons," in R. B. Helms, ed., Competitive Strategies in the Pharmaceutical Industry (Washington, D.C.: AEI Press, 1996).
- "Prescription Drug Pricing in the United States: Drug Companies Profit at the Expense of Older Americans," Minority Staff Report, Committee on Government Reform and Oversight, U.S. House of Representatives, October 20, 1998.
- General Accounting Office, "Drug Prices: Effects of Opening Federal Supply Schedule for Pharmaceuticals Are Uncertain," Washington, D.C., HEHS-97-60, June 11, 1997; www.gao.gov/AIndexFY97.
- General Accounting Office, "Medicaid: Changes in Best Price for Outpatient Drugs Purchased by HMOs and Hospitals," Washington, D.C., GAO/HEHS-94-194FS, 1994.
- Congressional Budget Office, "How the Medicaid Rebate on Prescription Drugs Affects Pricing in the Pharmaceutical Industry," Washington, D.C., 1996.
- See Mark E. Litow, "Vouchers as an Option in Medicare," Milliman & Robertson, Inc., and "Prescription Drugs for Seniors: Private Health Plans Could Provide Coverage at No Extra Cost," summary of findings of the Milliman & Robertson study, National Center for Policy Analysis, both forthcoming.
- Heinz Redwood, Price Regulation and Pharmaceutical Research (Suffolk, Eng.: Oldwicks Press, 1993); and David Gross et al., "International Spending Controls: France, Germany, Sweden and the United Kingdom," Health Care Financing Review, Vol. 15, No. 3, 1994, pp. 127-40.
- "ACE-Inhibitors Cost-Effective in CHF, " Scrip Magazine, No. 284, 1993, p. 60.
- C. Z. Louis et al., "Patterns of Hospital Care and Physician Perspectives from an Italian, Japanese and USA Hospital," American Journal of Medical Quality, Vol. 11, No. 3, Fall 1996, pp. 123-34.
- Lacy Glenn Thomas III, "Japan in Pharmaceuticals," in Pharmaceutical Benefit Scheme and Quality Care (Tokyo: Pfizer Health Research Foundation, 1995).
- Harry Schwartz, "National Health Insurance: Ill Health for the Industry?" Pharmaceutical Executive, March 1992, pp. 20-23.
- E. M. Kolassa, personal communication, August 24, 1999.
- A. J. Collins et al., "Trends in Anemia Treatment with Erythropoietin Usage and Patient Outcomes," American Journal of Kidney Disease, Vol. 32, No. 6, Supp. 4, December 1998, pp. S133-41.
- Ibid.
- S. B. Soumerai et al., "Effects of Medicaid Drug Payment Limits on Admissions to Hospitals and Nursing Homes," New England Journal of Medicine, Vol. 1, No. 2, October 10, 1991, p. 337.
- S. B. Soumerai et al., "Effects of a Limit on Medicaid Drug-Reimbursement Benefits on the Use of Psychotropic Agents and Acute Mental Health Services by Patients with Schizophrenia," New England Journal of Medicine, Vol. 331, No. 10, September 8, 1994, pp. 650-55.
- Lacy Glenn Thomas III, "Implicit Industrial Policy: The Triumph of Britain and the Failure of France in Global Pharmaceuticals, " School of Business, Emory University, February 1993.
- Ibid., page 15.
- "Italy, France Top 'Useless' Drug League," Scrip newsletter, October 28, 1993, p. 5.
- Ibid.
- Robert Chew, Senior Research Analyst, Association of the British Pharmaceutical Industry, personal communication, October 20, 1993.
- "German Brief," Chemist and Druggist, November 5, 1993, pp. 12-15.
- Henry G. Grabowski and John M. Vernon, "Returns on New Drug Introductions in the 1980s," American Enterprise Institute on Competition in the Pharmaceutical Industry, Duke University, October 27, 1993, Supp. Appendix, p. 3.
- Statement of Judith L. Wagner, Ph.D., Senior Associate, Health Program, Office of Technology Assessment, before the Special Committee on Aging, Washington, D.C., November 16, 1993, p. 13.
- Barbara Ryan, , Pharmaceutical Analyst, BTAlex Brown, presentation at Capitol Hill Briefing on Prescription Drug Price Regulation and Pharmaceutical Innovation, sponsored by the Ethics and Public Policy Center, June 14, Washington, D.C., 1999.
Robert Goldberg, Ph.D., is a Senior Research Fellow at the Ethics and Public Policy Center with the Center's project on Medical Science and Society. Dr. Goldberg has conducted research and written extensively on price controls on biopharmaceutical innovation, FDA control of medical information and the impact of government regulation of medicine on health care quality. His articles on health care and social policy have appeared in Reader's Digest, the Wall Street Journal, Washington Post, Los Angeles Times, Policy Review and Regulation. He is an adjunct scholar at the American Enterprise Institute. He received his Ph.D. in Public Policy at Brandeis University in 1984.